- Volumes 108-119 (2025)
-
Volumes 96-107 (2025)
-
Volume 107
Pages 1-376 (December 2025)
-
Volume 106
Pages 1-336 (November 2025)
-
Volume 105
Pages 1-356 (October 2025)
-
Volume 104
Pages 1-332 (September 2025)
-
Volume 103
Pages 1-314 (August 2025)
-
Volume 102
Pages 1-276 (July 2025)
-
Volume 101
Pages 1-166 (June 2025)
-
Volume 100
Pages 1-256 (May 2025)
-
Volume 99
Pages 1-242 (April 2025)
-
Volume 98
Pages 1-288 (March 2025)
-
Volume 97
Pages 1-256 (February 2025)
-
Volume 96
Pages 1-340 (January 2025)
-
Volume 107
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
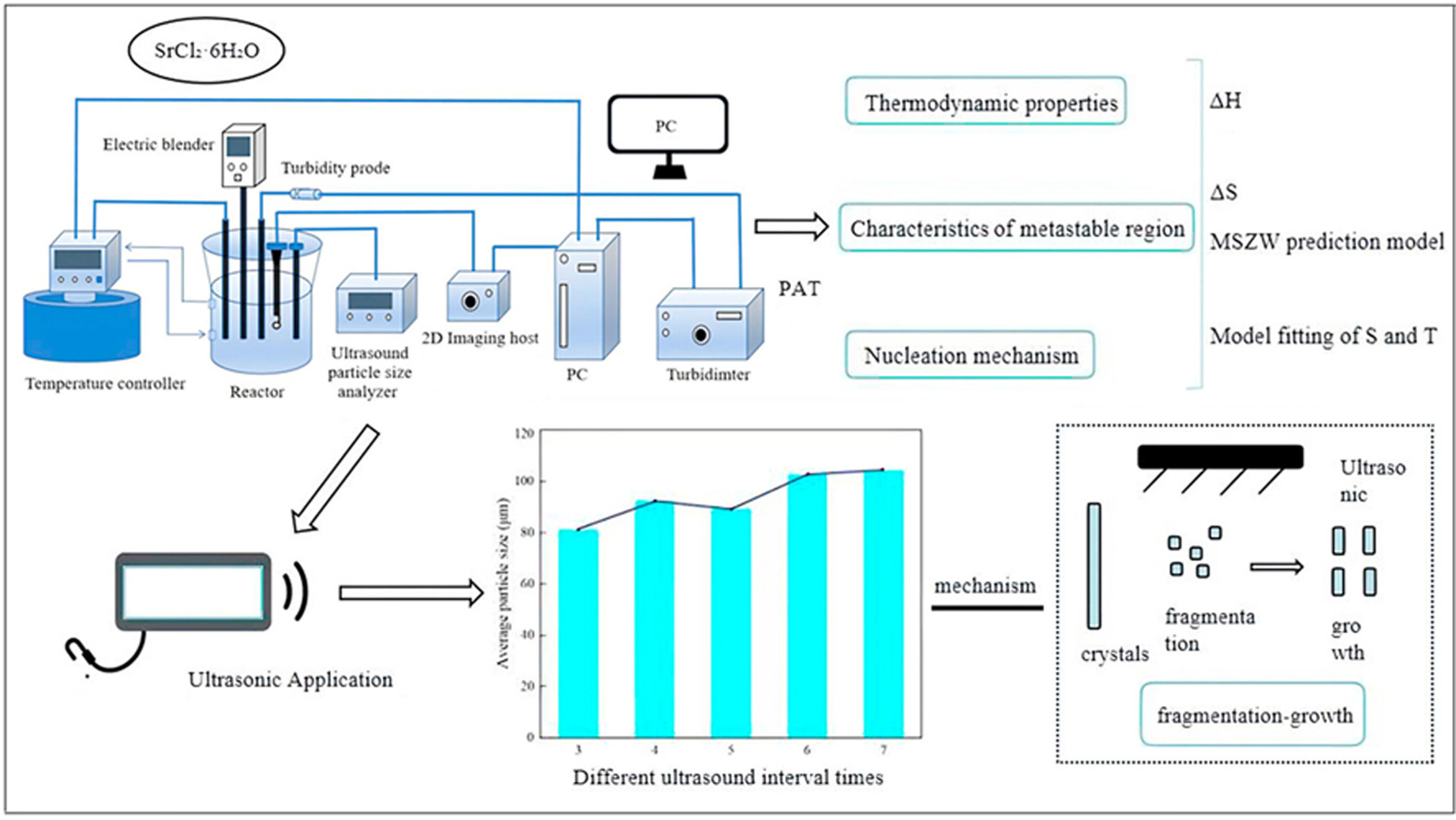
• Thermodynamic parameters of crystallization were derived and calculated through model fitting.
• A kinetic metastable zone prediction model was established based on nucleation theory.
• Anti-Caking performance of SrCl2·6H2O particles was significantly enhanced.
• This work provides a theoretical guidance for practical applications through crystallization theory.
Crystallization remains a fundamental separation and purification technique in chemical manufacturing. A comprehensive understanding of aqueous solution thermodynamics, metastable zone width (MSZW), and nucleation mechanisms is essential for optimizing crystallization processes, defining operational control parameters, and enabling subsequent crystal morphology control. This study systematically investigates the crystallization behavior of SrCl2·6H2O through in situ monitoring using process analytical technology (PAT). Key parameters, including MSZW, thermodynamic properties (solubility, supersaturation), and nucleation kinetics, were quantitatively determined to develop a predictive process model. To address the critical industrial challenge of product agglomeration arising from poor particle morphology, which complicates storage, transportation, and downstream processing while compromising product quality and increasing operational costs, an ultrasonic regulation strategy was implemented under optimized crystallization conditions. Post-treatment with optimized ultrasonic parameters yielded a marked reduction in particle aspect ratio, substantial improvement in dispersion, and a clear morphological transition from rod-like to granular crystals. This transformation significantly enhanced anti-agglomeration performance, thereby increasing product value. The regulatory mechanism of ultrasound is attributed to the "fragmentation-growth" mechanism, where ultrasonic cavitation induces controlled particle fragmentation followed by directional growth.