- Volumes 108-119 (2025)
-
Volumes 96-107 (2025)
-
Volume 107
Pages 1-376 (December 2025)
-
Volume 106
Pages 1-336 (November 2025)
-
Volume 105
Pages 1-356 (October 2025)
-
Volume 104
Pages 1-332 (September 2025)
-
Volume 103
Pages 1-314 (August 2025)
-
Volume 102
Pages 1-276 (July 2025)
-
Volume 101
Pages 1-166 (June 2025)
-
Volume 100
Pages 1-256 (May 2025)
-
Volume 99
Pages 1-242 (April 2025)
-
Volume 98
Pages 1-288 (March 2025)
-
Volume 97
Pages 1-256 (February 2025)
-
Volume 96
Pages 1-340 (January 2025)
-
Volume 107
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
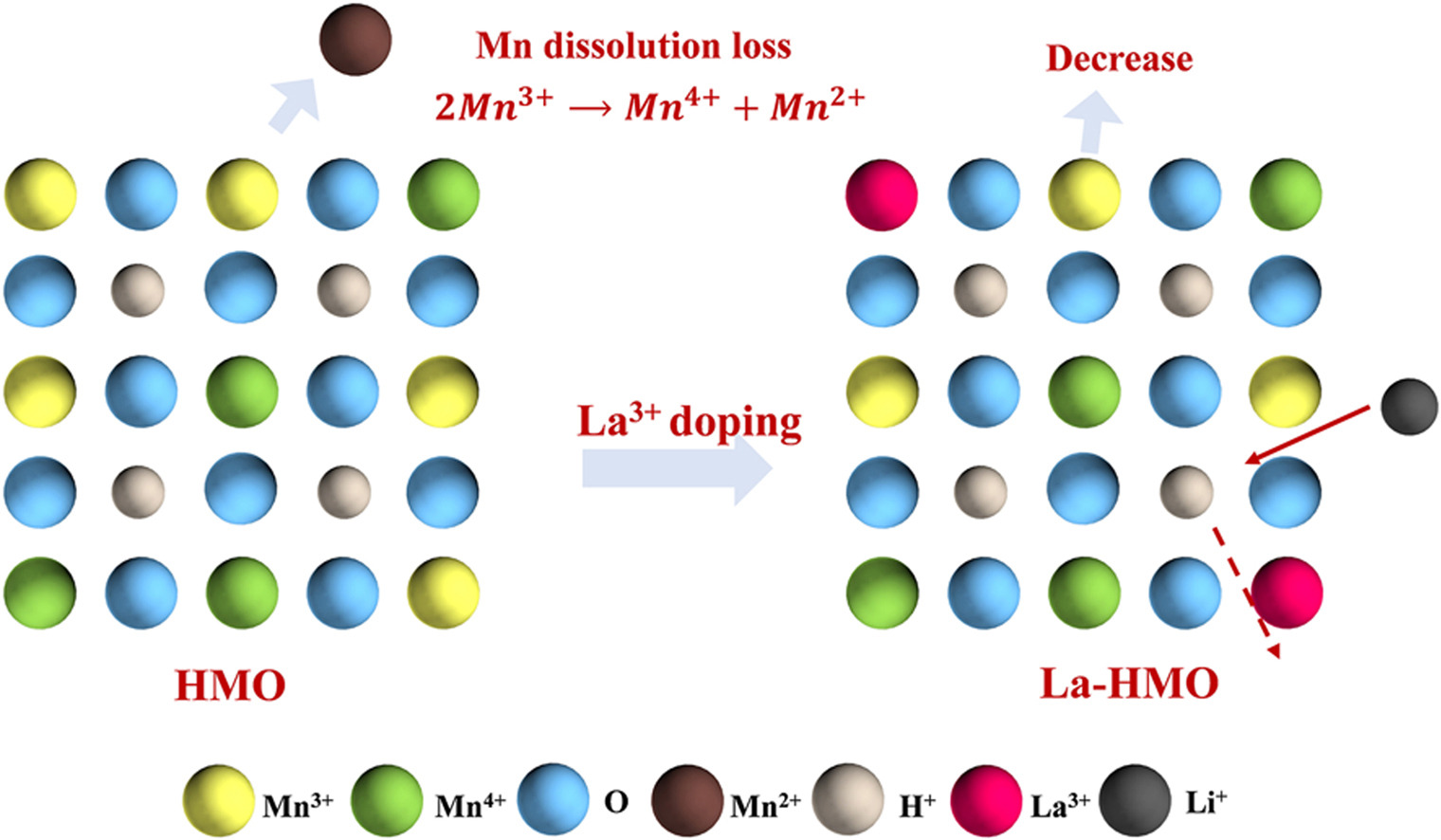
• La3+ doped spinel-type La-HMO lithium ion-sieve was easily synthesized.
• La3+ doping increases average oxidation state of Mn, effectively inhibits dissolution of Mn.
• After 5 cycles, Mn dissolution loss of La-HMO decreased from an initial 4.62 %–4.04 %.
Driven by the increasing global demand for lithium, significant attention has been directed toward developing efficient technologies for lithium extraction from Salt Lake brines. Li1.6Mn1.6O4 spinel shows high lithium selectivity and notable theoretical uptake capacity, indicating strong application potential in lithium extraction. However, the industrial application of this material is limited by its strong Jahn-Teller effect and manganese dissolution loss. In this study, LiOH and MnCO3 were selected as the raw materials and La2O3 was used as the doping modifier. The La3+-doped Li1.6Mn1.6O4 precursor (La-LMO) was fabricated through hydrothermal and high-temperature solid-phase synthesis techniques. Then La3+ doped H1.6Mn1.6O4 lithium ion-sieve (La-HMO) was prepared by pickling. The materials' structure and morphology were examined using XRD, SEM, TEM, and XPS techniques. La3+ doping does not alter the spinel structure of LMO but reduces Mn3+ content, mitigates the Jahn-Teller effect, lowers the manganese dissolution rate, and enhances structural stability. Adsorption of Li+ onto La-HMO follows pseudo-second-order kinetics and fits the Langmuir model, suggesting homogeneous monolayer adsorption driven by chemisorption. In the lithium extraction experiment from the brine of West Tai Kinel Salt Lake, La-HMO demonstrated high adsorption capacity and superior selectivity for Li+. After five cycles, La-HMO maintained an adsorption capacity of 33.10 mg/g, higher than the 26.80 mg/g for undoped HMO. The manganese dissolution rate dropped from 4.65 % to 4.04 %. The study significantly improved the adsorption properties and structural stability of HMO by doping La3+, which has broad application prospects in the separation and extraction of lithium resources in Salt Lake.