- Volumes 108-119 (2025)
-
Volumes 96-107 (2025)
-
Volume 107
Pages 1-376 (December 2025)
-
Volume 106
Pages 1-336 (November 2025)
-
Volume 105
Pages 1-356 (October 2025)
-
Volume 104
Pages 1-332 (September 2025)
-
Volume 103
Pages 1-314 (August 2025)
-
Volume 102
Pages 1-276 (July 2025)
-
Volume 101
Pages 1-166 (June 2025)
-
Volume 100
Pages 1-256 (May 2025)
-
Volume 99
Pages 1-242 (April 2025)
-
Volume 98
Pages 1-288 (March 2025)
-
Volume 97
Pages 1-256 (February 2025)
-
Volume 96
Pages 1-340 (January 2025)
-
Volume 107
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
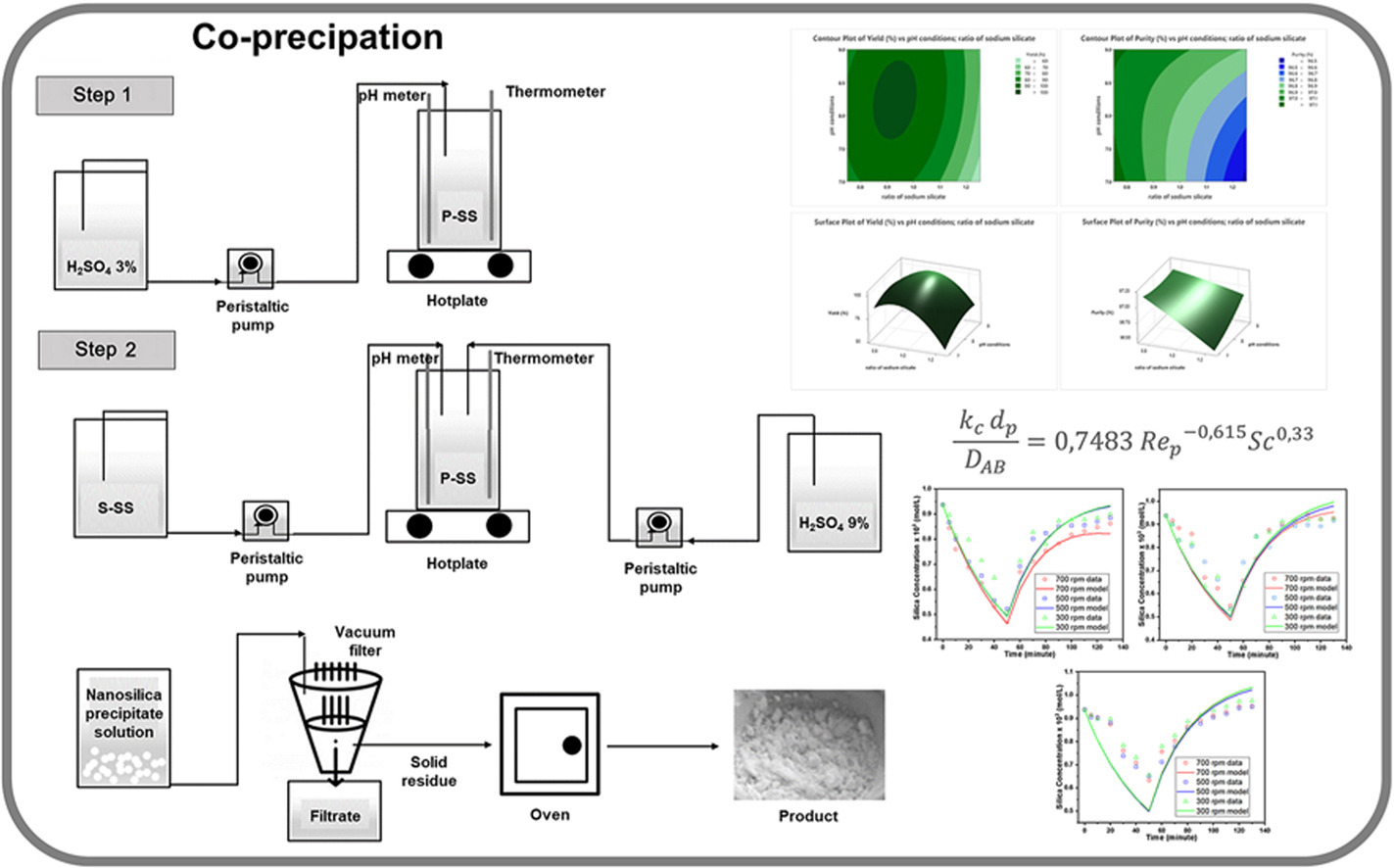
• Optimized co-precipitation yields high-purity silica nanoparticles from geothermal sludge.
• Process maximizes yield (100 %) and purity (97 %) while minimizing reagent use and complexity.
• Nanoparticles are amorphous, nanosized (10–100 nm), and suitable for industrial applications.
• Converts waste sludge (>80 % SiO2) into nanomaterials with ∼60–80 % higher market value.
• RSM–CCD modeling ensures process predictability, reproducibility, and sustainability.
Silica nanoparticles play a vital role in a range of industries, including agriculture, pharmaceuticals, biomedicine, ceramics, and advanced materials. However, conventional synthesis methods typically rely on expensive and environmentally burdensome chemical precursors. This study explores the hypothesis that geothermal sludge, a by-product of geothermal power plants, can serve as a sustainable and efficient source for producing high-purity silica nanoparticles through a simplified synthesis approach. The process involves three key purification stages—water washing, acid leaching, and conversion to sodium silicate—followed by co-precipitation to obtain the final silica product. To optimize the synthesis, experimental conditions were statistically evaluated using response surface methodology to identify the effect of pH and sodium silicate ratio on the silica yield. The highest yield (100 %) and purity (97.03 %) were achieved under neutral pH conditions and a sodium silicate ratio of 1:1 by volume. Material characterization was conducted using elemental analysis, X-ray diffraction, and electron microscopy to confirm the structural and morphological properties. In addition, kinetic modeling revealed the influence of agitation speed and temperature on silica precipitation dynamics, and a dimensionless correlation was developed to quantify the mass transfer coefficient during the process. The findings demonstrate a promising and more sustainable pathway for silica nanoparticle production using industrial waste as feedstock. While direct financial metrics were not assessed, the use of readily available by-products and simplified process conditions suggest potential for economic advantages, meriting further techno-economic evaluation in future work.