- Volumes 96-107 (2025)
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
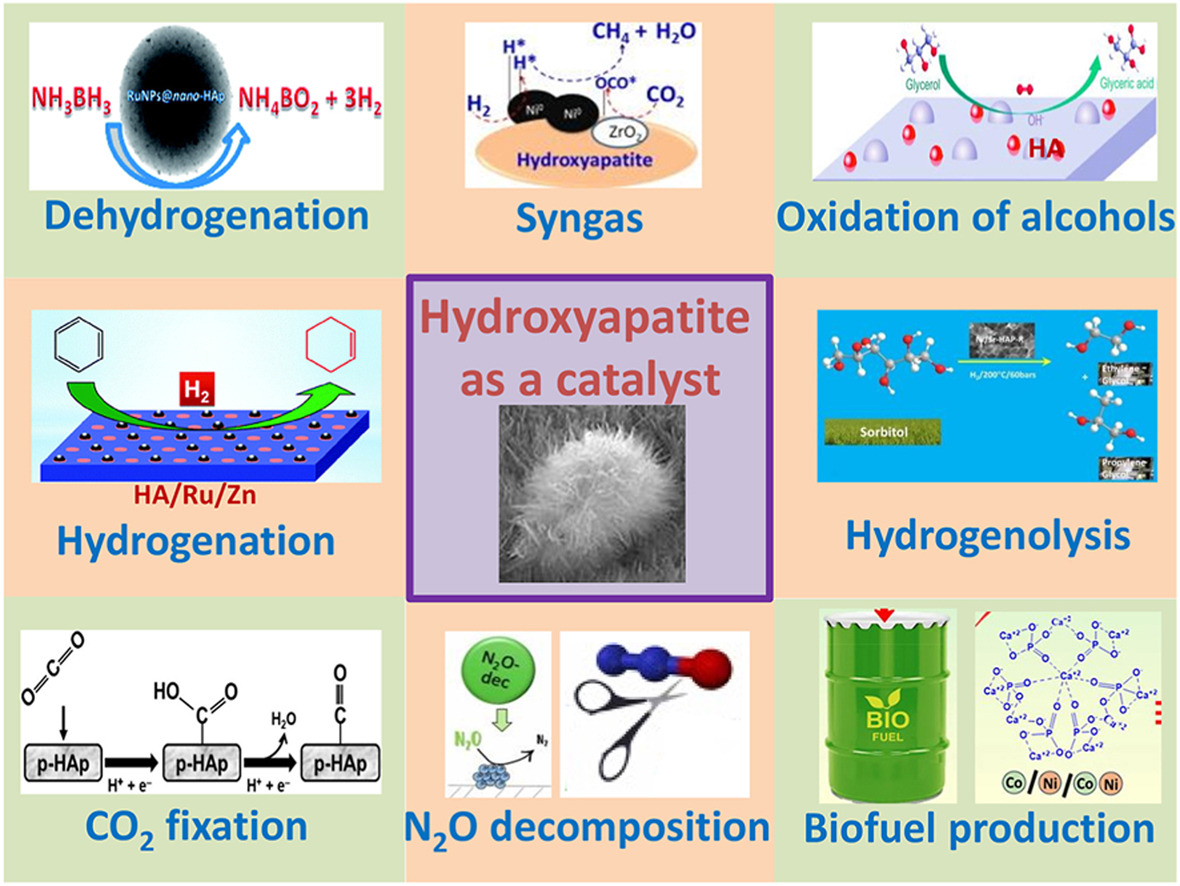
• Hydroxyapatite both in free and doped forms can be used as a catalyst.
• Hydroxyapatite catalysts are prepared by impregnation or co-precipitation.
• Oxidation of alcohols and C-C bond formation are main HA catalytic uses.
• Low- and large-scale organic processes can be HA-catalyzed.
• CO2 fixation and N2O decomposition are frequently HA-catalyzed.
Hydroxyapatite, a bioceramic material, possesses sufficient stability in aqueous and organic medium and it is mainly known for its role in tooth and bone structure. However, it is less-known that, in certain conditions, the HA can be used as a catalyst in its free, metal-doped and composite forms. HA as a catalyst is generally prepared by i mpregnation or co-precipitation methods, sometimes from natural P-containing wastes. HA can be doped with mono- or polymetallic ions or nanoparticles and can contain other admixtures or supports (i.e., carbon). Different Ca/P ratios (ideal 1.67; the ranges are 1.5–1.7 in synthetic and 1.5–1.8 in biologic forms) can be revealed for the HA. Nanosized HA forms are frequent in several applications. A variety of distinct processes can be HA-catalyzed, such as oxidation of alcohols, dehydrogenation, hydrogenation and hydrogenolysis, C-C bond formation, among other important existing low- and large-scale organic processes. The HA can be also used for catalytic environmental remediation, CO2 fixation, and N2O decomposition. In this review, we emphasize most recent advances (mainly last decade) on the catalytic HA applications, except for biomedical ones.