• A switch controllable NPF/D system improves the tumor homing and bioavailability of chemotherapy drugs.
• Fluorescence and toxicity only can be activated within tumor cells instead of normal cells.
• NPF/D avoided DOX efflux to overcome chemoresistance.
• NPF/D exhibited remarkably primary and distant tumor suppression.
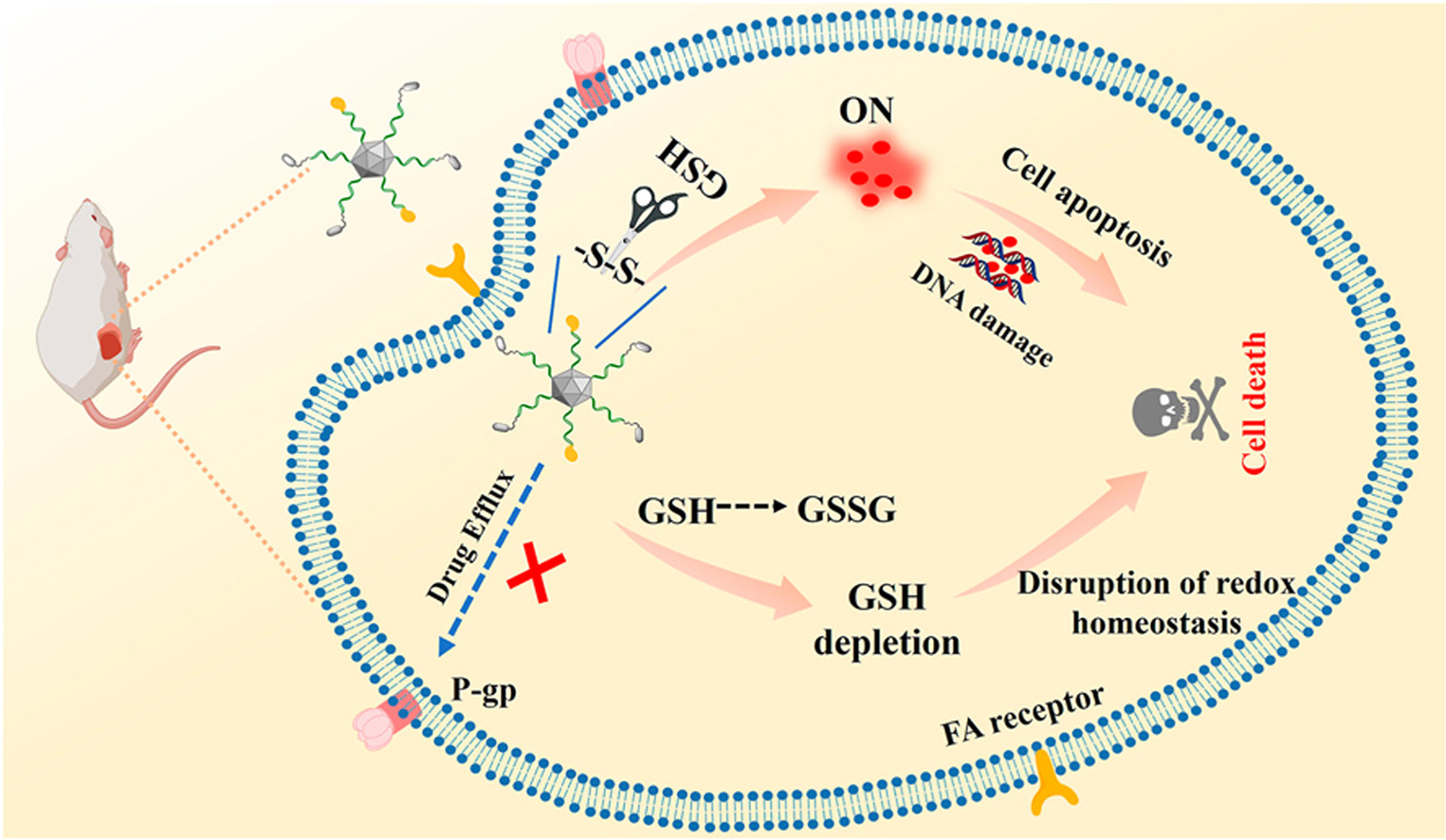
Many small molecule chemotherapy drugs suffer from the disadvantages of poor water solubility, tumor off-target and resistance to chemotherapeutics, resulting in severe toxic side effects. Herein, a folic acid-targeted nanodiamond drug delivery system (NPF/D) was fabricated. The fluorescence of doxorubicin (DOX) is quenched when DOX is immobilized on the nanodiamond (ND) through disulfide bonds, which can be broken by overexpressed endogenous glutathione (GSH) within tumor cells to lead to drug release and GSH depletion, thereby selectively killing cancer cells instead of normal cells and increasing cancer cells sensitivity to chemotherapy drugs. In addition, the NPF/D system can overcome drug efflux and improve the efficacy of chemotherapy against drug resistance. It was of great importance that NPF/D effectively accumulated at the tumor site and significantly inhibited tumor growth, where the tumor volume was reduced by about 3 times compared to the control group. Interestingly, the combination of NPF/D with Toll-like receptor 7 agonist imiquimod (R837) and programmed cell death ligand 1 antibody (anti-PD-L1) markedly inhibited distant tumor growth, indicating good immune response. Therefore, such a nanodiamond nanoplatform with the integration of various functions has successfully demonstrated its promise for safe and efficient cancer treatment.