- Volumes 108-119 (2025)
-
Volumes 96-107 (2025)
-
Volume 107
Pages 1-376 (December 2025)
-
Volume 106
Pages 1-336 (November 2025)
-
Volume 105
Pages 1-356 (October 2025)
-
Volume 104
Pages 1-332 (September 2025)
-
Volume 103
Pages 1-314 (August 2025)
-
Volume 102
Pages 1-276 (July 2025)
-
Volume 101
Pages 1-166 (June 2025)
-
Volume 100
Pages 1-256 (May 2025)
-
Volume 99
Pages 1-242 (April 2025)
-
Volume 98
Pages 1-288 (March 2025)
-
Volume 97
Pages 1-256 (February 2025)
-
Volume 96
Pages 1-340 (January 2025)
-
Volume 107
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
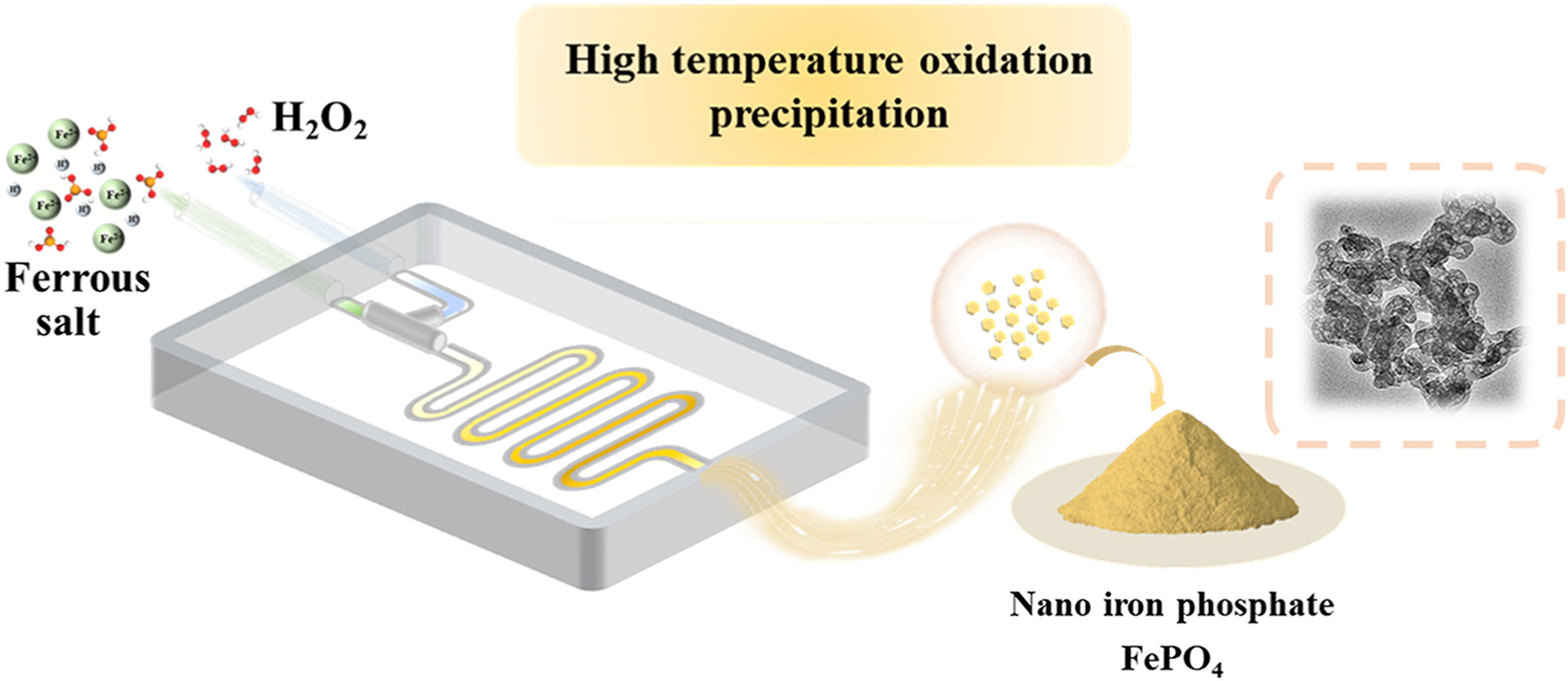
• Established a microflow system for high-temperature precipitation of nano FePO4.
• Proposed a novel high temperature oxidation precipitation method for synthesizing high-purity nano-sized ferric phosphate.
• Illustrated effect of high temperature on surface hydroxyl groups of particles and properties of the slurry.
• The as-prepared LiFePO4@C material exhibited excellent rate performance (137.1 mA h g−1 at 5 C).
A novel high temperature oxidation precipitation method for synthesizing high-purity nano-sized ferric phosphate was proposed. We systematically investigated the impact of reaction temperature on the properties of the ferric phosphate product and its slurry. The study found that the FePO4 obtained by the high-temperature precipitation method consisted of amorphous nanoparticles with a narrow size distribution around 30 nm. Increasing the reaction temperature did not affect the purity or crystal structure of the particles, but it reduced the viscosity and solid content of the slurry, beneficial for improving the solid-liquid separation efficiency in subsequent production processes. Characterization of products obtained at different reaction temperatures using FTIR, XPS, and ICP-OES revealed that elevated temperatures decreased the content of hydroxyl groups on the surface of the ferric phosphate particles, weakening the adsorption of metal ion impurities on the particle surface and the interaction between particles. The LiFePO4@C material synthesized using the nano FePO4 product obtained by the high-temperature oxidative precipitation method as a precursor exhibited good rate performance (137.1 mAh g−1 at 5 C). This high-temperature oxidative precipitation method might enable controllable, continuous, and easily scalable production of nano-sized FePO4 production.