- Volumes 108-119 (2025)
-
Volumes 96-107 (2025)
-
Volume 107
Pages 1-376 (December 2025)
-
Volume 106
Pages 1-336 (November 2025)
-
Volume 105
Pages 1-356 (October 2025)
-
Volume 104
Pages 1-332 (September 2025)
-
Volume 103
Pages 1-314 (August 2025)
-
Volume 102
Pages 1-276 (July 2025)
-
Volume 101
Pages 1-166 (June 2025)
-
Volume 100
Pages 1-256 (May 2025)
-
Volume 99
Pages 1-242 (April 2025)
-
Volume 98
Pages 1-288 (March 2025)
-
Volume 97
Pages 1-256 (February 2025)
-
Volume 96
Pages 1-340 (January 2025)
-
Volume 107
-
Volumes 84-95 (2024)
-
Volume 95
Pages 1-392 (December 2024)
-
Volume 94
Pages 1-400 (November 2024)
-
Volume 93
Pages 1-376 (October 2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 95
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
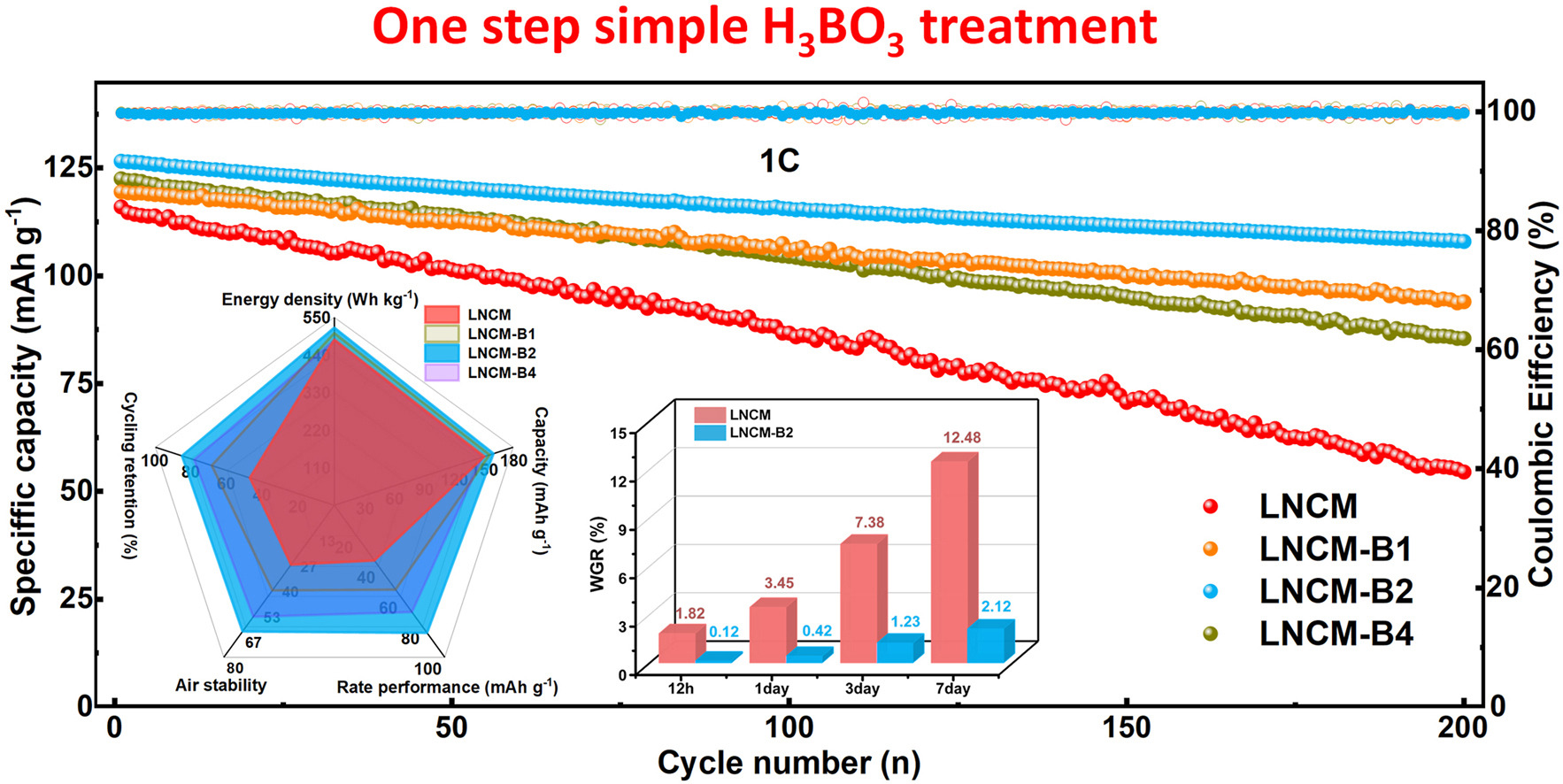
• A one-step H3BO3 treatment introduces near-surface B doping and sodium borate interphase.
• Near-surface B doping reinforces the layered structure and suppresses irreversible oxygen redox.
• Sodium borate interphase ensures fast Na+ transport while mitigating electrolyte decomposition and TM dissolution.
• Optimized cathode exhibits superior electrochemical performance and exceptional air stability.
• Providing a practical pathway for achieving electrochemically robust and moisture-tolerant sodium-ion cathodes.
O3-type layered oxides are among the most promising cathode candidates for sodium-ion batteries, yet their practical use is constrained by irreversible oxygen redox at high voltages, parasitic side reactions, and pronounced moisture sensitivity. Here, we propose a one-step H3BO3 treatment that introduces an in-situ boride complex, enabling near-surface B doping and the formation of a conformal sodium borate interphase. The dual modification operates synergistically: B incorporation reinforces the layered framework and suppresses oxygen redox above 4.0 V, while the sodium borate layer, endowed with high Na+ conductivity, functions as a robust interfacial barrier. These effects collectively suppress transition-metal dissolution, mitigate electrolyte decomposition, and promote rapid Na+ transport. Benefiting from this design, the optimized cathode delivers 160.5 mAh g−1 at 0.1 C and retains 85.3 % capacity after 200 cycles at 1 C. Moreover, the sodium borate coating effectively blocks H+/H2O ingress, conferring exceptional air stability. After 150 cycles, 3-days-aged pristine cathode retains 4.7 % of the fresh capacity, whereas optimized cathode maintains nearly pristine cycling stability. Even after seven days of exposure, only trace Na2CO3 impurities are detected. This work establishes in situ boride complexes as a viable strategy to achieve electrochemically robust and moisture-tolerant sodium-ion cathodes for grid-scale energy storage.